
Welcome to This Blog Has Integrity
In a world where artificial intelligence, computerized systems, and regulations evolve rapidly, data reliability and completeness have become the foundation o...
Professional insights, best practices, and regulatory updates in Data Integrity and Computerized System Validation (CSV).
The posts and articles reflect practical perspectives inspired by the CPF approach, integrating quality, compliance, and innovation.

Want to strengthen your brand on LinkedIn? Or on social media in general? It all starts with the reliability of the information you share. I often read tips a...

Today I met a colleague, and we ended up talking about the use of AI in analytical method development, among other things. At the same time, quite a few posts o...

How much I love that moment when the token drops — you know, like the quarter in old public payphones. And yes — for the younger generation, that simply means “...

🎮 Tetris or Puzzle – which one fits your world better? Or maybe it’s the same… because none of us really have time for either 😉 In our field, though, the diff...

🚀 “Less paperwork, more assurance.” The FDA’s final Computer Software Assurance (CSA) guidance is here — and it’s reshaping how we approach computerized s...

🚦 Do you really know which systems manage your critical data? Most organizations are confident they do — until an auditor shows up and asks one simple question...

🌞 Nothing New Under the Sun – Just New Technology Around Us Lately, I’ve noticed more and more organizations (still a small minority, though) starting to draf...

Before founding my consulting firm, I was responsible for laboratory systems in a pharmaceutical company. Among my key roles was bridging between the business —...

About eight years ago, I was asked a question that has stayed with me ever since. And today, in a completely different context, I found myself returning to tha...
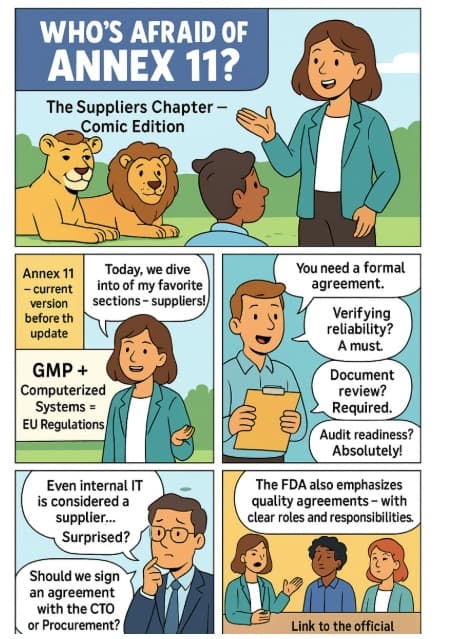
Annex 11 – Part 5 Or in other words: Another episode in the series "Who’s Afraid of Annex 11?" This time, we dive into one of the hottest topics among my collea...

Annex 11 – Part 4 Or in other words: Another episode in the series "Who’s Afraid of Annex 11?" This time we focus on one of my favorite and most formative parag...

🧭 Risk Management in Computerized Systems – A Core Principle of Annex 11 Quality control in computerized systems requires a structured, risk-based approach. T...

The Annex 11 document defines the #GMP requirements for computerized systems and forms part of the European quality-management regulations for the pharmaceuti...
Who’s Afraid of Annex 11? Part One This series will explore the subtopics related to the requirements set by the EMA – the European Medicines Agency , which is...

Five years ago, I wrote a post about my son – back then, a young superhero fan deeply immersed in Rick Riordan’s books. He’s grown since then, but one thing ha...

This time, I want to talk about one of the most powerful tools we have – and the one you’ll never find in any procedure or SOP: 🗣️ Dialogue. Whenever we dis...

It’s widely accepted today that #Data is one of our most valuable resources. Every decision we make — big or small — relies on information derived from data....
You Don't Change the Oil Filter on a Parked Car A brilliant quote (not mine!) from my outsourcing partner, during a deep discussion on UAT — User Acceptance Tes...

In every project, open issues arise. They’re not necessarily risks – but if left unresolved, they might become one. Not long ago, I sat with a frustrated proje...
Disclaimer: The content reflects professional knowledge and experience only and does not constitute specific advice. Each organization should perform its own professional assessment before implementation.